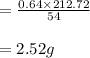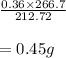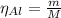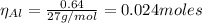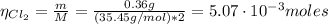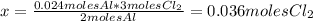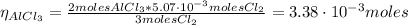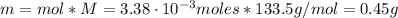Chemistry, 25.03.2020 05:47 ruchierosanp1n3qw
The balanced equation for the reaction of aluminum metal and chlorine gas is 2Al(s) + 3Cl2(g) → 2AlCl3(s) Assume that 0.64 g Al is mixed with 0.36 g Cl2. (a) What is the limiting reactant? Al Cl2 (b) What is the maximum amount of AlCl3, in grams, that can be produced?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:50, stephaniero6
2points why do scientists need governmental funding? o a. government politicians ask all the important scientific questions. o b. scientists have to pay taxes to the government on the money they make. o c. the cost of doing scientific research can be very high. o d. the government is controlled by scientists. submit
Answers: 3


Chemistry, 22.06.2019 08:30, Blaise2653
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 22.06.2019 10:30, kdenormandie3122
Geothermal energy for industrial use is available almost anywhere. a. true b. false
Answers: 2
You know the right answer?
The balanced equation for the reaction of aluminum metal and chlorine gas is 2Al(s) + 3Cl2(g) → 2AlC...
Questions in other subjects:



English, 05.05.2020 02:55


Mathematics, 05.05.2020 02:55




Mathematics, 05.05.2020 02:55

Mathematics, 05.05.2020 02:55