
Chemistry, 25.03.2020 04:59 levelebeasley1
Suppose a 1.0 L solution contains 0.020 M in Cu(NO3)2, then 0.40 moles of NH3 are added. Assuming no change in volume, what is the concentration of Cu2+ ions in the solution after the addition of NH3? The Kf for Cu(NH3)42+ is 1.7x1013.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:10, Tyrant4life
Which of these is the result of scientific research and not engineering? a. a new shoe design that features air cushioning for more comfort and protection b. the creation of glass with uv protection. c. a conclusion about diet commonalities among diabetics. d. the development of a smaller, more compact missile.
Answers: 1


Chemistry, 22.06.2019 10:00, ellaemtagedeane
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 12:00, carvajalj2520
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1
You know the right answer?
Suppose a 1.0 L solution contains 0.020 M in Cu(NO3)2, then 0.40 moles of NH3 are added. Assuming no...
Questions in other subjects:

Mathematics, 07.07.2019 09:30



Mathematics, 07.07.2019 09:30

Mathematics, 07.07.2019 09:30



Biology, 07.07.2019 09:30


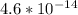 M
M![Cu^{2+} = [Cu(NO_3)_2]](/tpl/images/0562/4161/18067.png) = 0.020 M
= 0.020 M![Cu^{2+}+4NH_3_{aq} \rightleftharpoons [Cu(NH_3)_4]^{2+}_{(aq)}](/tpl/images/0562/4161/56ee7.png)
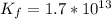
![K_f } = \frac{[Cu(NH_3)_4]^{2+}}{[Cu^{2+}][NH_3]^4}](/tpl/images/0562/4161/1ed4c.png)
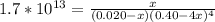
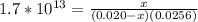
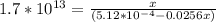
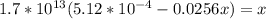
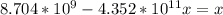
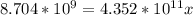
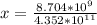
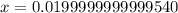
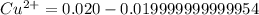
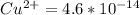 M
M 

