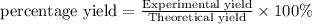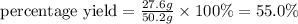Chemistry, 25.03.2020 04:57 trevorhenyan51
Ammonia gas can be prepared by the reaction CaO ( s ) + 2 NH 4 Cl ( s ) ⟶ 2 NH 3 ( g ) + H 2 O ( g ) + CaCl 2 ( s ) In an experiment, 27.6 g of ammonia gas, NH 3 , is produced when it was predicted that 50.2 g NH 3 would form.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, gracelanghorn
Classify each statement about effective nuclear charge, zeff, as true or false.
Answers: 2

Chemistry, 22.06.2019 00:30, BLASIANNkidd
Maria wants to determine which type of disinfectant kills the most bacteria. which of the following is the best way for maria to determine this? a. ask ten different companies that make disinfectants which type is best. b. put the same amount and species of bacteria on ten identical plates, and add ten different kinds of disinfectant to each plate. c. interview ten different people to determine which type of disinfectant they prefer. d. put the same amount and species of bacteria on ten identical plates, and add a different disinfectant to each plate.
Answers: 1

Chemistry, 22.06.2019 03:30, fbillinton
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 16:00, hjgjlgkjg
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1
You know the right answer?
Ammonia gas can be prepared by the reaction CaO ( s ) + 2 NH 4 Cl ( s ) ⟶ 2 NH 3 ( g ) + H 2 O ( g )...
Questions in other subjects:



Mathematics, 05.03.2021 01:30



Mathematics, 05.03.2021 01:30

Mathematics, 05.03.2021 01:30

Chemistry, 05.03.2021 01:30


Mathematics, 05.03.2021 01:30

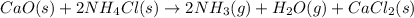
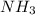 , is produced when it was predicted that 50.2 g of
, is produced when it was predicted that 50.2 g of