
Chemistry, 25.03.2020 03:45 kasier4600
Without performing any calculations, determine whether the entropy of the system increases or decreases in the photosynthesis of glucose: 6 CO2(g) + 6 H2O(ℓ) → C6H12O6(s) + 6 O2(g) 1. The entropy does not change. 2. decreases 3. increases 4. Unable to determine

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 23:40, tilievaughn14
What energy conversion occurs when a sling shot is used to shoot a rock across the room? (2 points) question 2 options: 1) stored mechanical energy is converted to mechanical energy. 2) stored mechanical energy is converted to radiant energy. 3) gravitational energy is converted to radiant energy. 4) gravitational energy is converted to mechanical energy.
Answers: 1

Chemistry, 23.06.2019 01:00, davelopez979
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1
You know the right answer?
Without performing any calculations, determine whether the entropy of the system increases or decrea...
Questions in other subjects:


Business, 17.12.2021 14:00

History, 17.12.2021 14:00

English, 17.12.2021 14:00

Computers and Technology, 17.12.2021 14:00

English, 17.12.2021 14:00


Mathematics, 17.12.2021 14:00

Mathematics, 17.12.2021 14:00

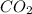 from the atmosphere and water from soil and finally make a highly ordered molecule Glucose, which is further used as a food product and source of energy by the plants. Thus it is clear that degree of randomness in decreasing in the process of photosynthesis and it is a negative change in entropy.
from the atmosphere and water from soil and finally make a highly ordered molecule Glucose, which is further used as a food product and source of energy by the plants. Thus it is clear that degree of randomness in decreasing in the process of photosynthesis and it is a negative change in entropy.


