Chemistry, 25.03.2020 03:05 JakkuZakku9436
Octane (C8H18) undergoes combustion according to the following thermochemical equation. 2C8H18(l) + 25O2(g) → 16CO2(g) + 18H2O(l) ΔH°rxn = –1.0940 × 104 kJ/mol What is the standard enthalpy of formation of liquid octane? ΔH°f(CO2(g)) = –393.5 kJ/mol and ΔH°f(H2O(l)) = –285.8 kJ/mol

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, thebrain1345
Plz me get these answer dubble cheak ur answer plz ppl i need it right
Answers: 2


Chemistry, 23.06.2019 13:30, michelle8978
Why hydrochloric acid neutralized first when you titrate a mixture of hcl& ch3cooh against standard sodium hydroxide
Answers: 1
You know the right answer?
Octane (C8H18) undergoes combustion according to the following thermochemical equation. 2C8H18(l) +...
Questions in other subjects:

Physics, 01.03.2021 09:00

Mathematics, 01.03.2021 09:00

Computers and Technology, 01.03.2021 09:00

Social Studies, 01.03.2021 09:00

English, 01.03.2021 09:00

Geography, 01.03.2021 09:00




Mathematics, 01.03.2021 09:00

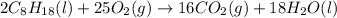
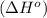 .
.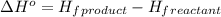
![\Delta H^o=[n_{O_2}\times \Delta H_f^0_{(O_2)}+n_{H_2O}\times \Delta H_f^0_{(H_2O)}]-[n_{C_8H_{18}}\times \Delta H_f^0_{(C_8H_{18})+n_{O_2}\times \Delta H_f^0_{(O_2)}]](/tpl/images/0562/3120/bdc44.png)
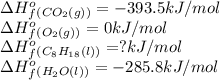
![-1.0940\times 10^4=[(16\times -393.5)+(18\times -285.8)]-[(25\times 0)+(2\times \Delat H_f{C_8H_{18}(l)}]](/tpl/images/0562/3120/9e959.png)