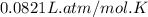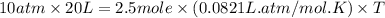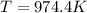A gaseous mixture is composed of 1.5 moles of He, 2.5 moles of N2 and an unknown number of moles of He. The mixture is contained within a 20-liter vessel. If the partial pressure of the N2 is 10 atm, what is the temperature of the mixture in degrees Kelvin, ˚K? R= 0.0821 L x atm/mol x ˚K.

Answers: 2


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 09:00, wkalpakchi
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1
You know the right answer?
A gaseous mixture is composed of 1.5 moles of He, 2.5 moles of N2 and an unknown number of moles of...
Questions in other subjects:

Mathematics, 18.05.2021 08:00


Social Studies, 18.05.2021 08:00


Mathematics, 18.05.2021 08:00



Mathematics, 18.05.2021 08:00

Mathematics, 18.05.2021 08:00

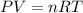
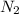 gas = 10 atm
gas = 10 atm