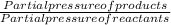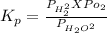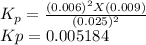The elementary reaction 2 H 2 O ( g ) − ⇀ ↽ − 2 H 2 ( g ) + O 2 ( g ) proceeds at a certain temperature until the partial pressures of H 2 O , H 2 , and O 2 reach 0.025 atm, 0.0060 atm, and 0.0090 atm, respectively at equilibrium. What is the value of the equilibrium constant at this temperature?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, yousifgorgees101
The earth's moon is unusually large. two popular theories of the moon's origin include the "sister world" hypothesis, which states that the moon formed from the same materials as the earth, near enough to the earth that they fell into orbit around each other. a second theory is the "capture" hypothesis, in which the moon formed elsewhere in the solar system, and the earth's gravity pulled it into its orbit. studies of what the moon is made of indicate that some of its materials had to come from the earth or from the same area of the solar system where the earth had formed. at the same time, the moon does not contain much of the material that makes up the earth's core, so the moon could not have formed from the same materials as the earth. how do the two facts above affect the described theories of the moon's origin? a. they show that scientists will never agree on where the moon came from. b. they show that more experiments on moon formation need to be done. c. they show that no theory accounts for the existence of the moon. d. they show that neither theory is complete and entirely correct.
Answers: 1

Chemistry, 22.06.2019 08:30, breannaking9734
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 20:00, emilyswinge4421
Listenbase your answer to the question on the information below. nuclear radiation is harmful to living cells, particularly to fast-growing cells, such as cancer cells and blood cells. an external beam of the radiation emitted from a radioisotope can be directed on a small area of a person to destroy cancer cells within the body. cobalt-60 is an artificially produced radioisotope that emits gamma rays and beta particles. one hospital keeps a 100.0-gram sample of cobalt-60 in an appropriate, secure storage container for future cancer treatment. which choice represents the correct product for the beta decay of the co-60? fe-60ni-60fe-61ni-61
Answers: 2

Chemistry, 23.06.2019 04:00, izzyp619
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3
You know the right answer?
The elementary reaction 2 H 2 O ( g ) − ⇀ ↽ − 2 H 2 ( g ) + O 2 ( g ) proceeds at a certain temperat...
Questions in other subjects:

History, 01.04.2020 04:12

History, 01.04.2020 04:12


Mathematics, 01.04.2020 04:12

History, 01.04.2020 04:12



English, 01.04.2020 04:12

Mathematics, 01.04.2020 04:12