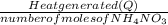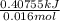Chemistry, 25.03.2020 02:03 AkramMasoud
Calculate the change in enthalpy for the reaction. (Use 1.0 g/mL as the density of the solution and 4.18 J/(g⋅∘C) as the specific heat capacity.) Express the enthalpy change in kilojoules per mole to two significant figures. ΔHrxn = nothing kJ/mol

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 20.06.2019 18:04, naynay4evr
What is the smallest component or the most basic building block of any element ? a. an atom, b. a compound c. gas d. element
Answers: 1

Chemistry, 22.06.2019 13:00, aleilyg2005
If two objects at different te, peraure are in contact with each other what happens to their temperature
Answers: 1


Chemistry, 22.06.2019 22:30, gonzalesalexiaouv1bg
What if it is did darwin used to support his theory of evolution
Answers: 1
You know the right answer?
Calculate the change in enthalpy for the reaction. (Use 1.0 g/mL as the density of the solution and...
Questions in other subjects:





Chemistry, 16.04.2021 19:40





Mathematics, 16.04.2021 19:40

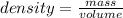
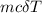
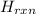 =
=