
Chemistry, 25.03.2020 01:35 naiomireyes74p2aybs
An element has the following successive ionization energies: I1 = 578 kJ/mol I2 = 1817 kJ/mol I3 = 2745 kJ/mol I4 = 11,577 kJ/mol I5 = 14,125 kJ/mol Based on these relative values, which ionization energies correspond to removing core (nonvalence) electrons?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, roseemariehunter12
When the earth was formed and cooled, why did nickel and iron end up in the center of the earth while basalt and granite ended up in the outer layers
Answers: 3

Chemistry, 22.06.2019 07:00, vivianni0727p1y30v
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 12:30, MrSavannahCat
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 14:00, luisaareli6298
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1
You know the right answer?
An element has the following successive ionization energies: I1 = 578 kJ/mol I2 = 1817 kJ/mol I3 = 2...
Questions in other subjects:


Arts, 04.06.2021 01:00






Social Studies, 04.06.2021 01:00

English, 04.06.2021 01:00

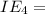 11,577 kJ/mol
11,577 kJ/mol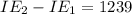 kJ/mol
kJ/mol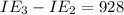 kJ/mol
kJ/mol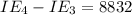 kJ/mol
kJ/mol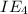 indicates the removal of core electron.
indicates the removal of core electron.


