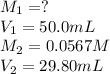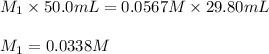Chemistry, 25.03.2020 00:28 itzdulceee
Acetic acid (CH3CO2H) is the principal component in the vinegar. What is the molarity of an acetic acid solution if a titration of 50.00 mL of the acetic acid solution requires 29.80 mL of 0.0567 M NaOH to reach the equivalence point?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, amylumey2005
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2


Chemistry, 22.06.2019 19:00, miguel454545
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 22.06.2019 23:00, lufung8627
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
You know the right answer?
Acetic acid (CH3CO2H) is the principal component in the vinegar. What is the molarity of an acetic a...
Questions in other subjects:





Mathematics, 24.04.2020 23:35


Mathematics, 24.04.2020 23:35

Mathematics, 24.04.2020 23:35

English, 24.04.2020 23:35

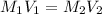
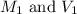 are the molarity and volume of acetic acid.
are the molarity and volume of acetic acid.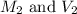 are the molarity and volume of NaOH.
are the molarity and volume of NaOH.