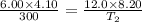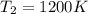Chemistry, 24.03.2020 23:00 charlesiarenee0
One mole of oxygen gas is at a pressure of 6.00 atm and a temperature of 27.0°C. (a) If the gas is heated at constant volume until the pressure triples, what is the fi nal temperature? (b) If the gas is heated so that both the pressure and volume are doubled, what is the fi nal temperature?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, dante766
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1


Chemistry, 22.06.2019 20:00, Isaiahtate053
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

Chemistry, 23.06.2019 00:10, Rubendelarosa1529
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3
You know the right answer?
One mole of oxygen gas is at a pressure of 6.00 atm and a temperature of 27.0°C. (a) If the gas is h...
Questions in other subjects:




Health, 11.03.2021 21:20




Mathematics, 11.03.2021 21:20


Mathematics, 11.03.2021 21:20

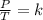
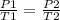
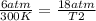
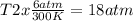
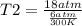
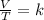
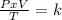
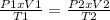
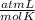 × 300 K
× 300 K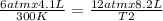
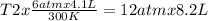
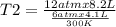
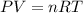
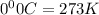
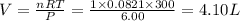
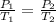
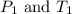 are the initial pressure and temperature of the gas.
are the initial pressure and temperature of the gas.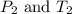 are the final pressure and temperature of the gas.
are the final pressure and temperature of the gas.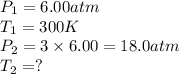
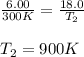
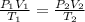
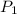 = initial pressure of gas = 6.00 atm
= initial pressure of gas = 6.00 atm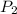 = final pressure of gas =
= final pressure of gas = 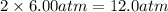
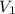 = initial volume of gas = 4.10 L
= initial volume of gas = 4.10 L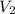 = final volume of gas =
= final volume of gas = 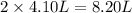
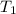 = initial temperature of gas =
= initial temperature of gas = 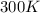
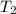 = final temperature of gas =?
= final temperature of gas =?