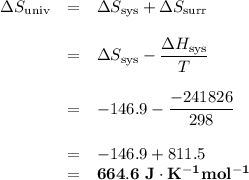Calculate the ΔH, ΔS and ΔSuniverse for this reaction.

Chemistry, 24.03.2020 23:01 Cartucho1978
½O2(g) + H2(g) ⇌ H2O(g)
Calculate the ΔH, ΔS and ΔSuniverse for this reaction.
Determine the spontaneity of the reaction.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 20:20, catchonyet
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 23.06.2019 03:50, mobslayer88
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2
You know the right answer?
½O2(g) + H2(g) ⇌ H2O(g)
Calculate the ΔH, ΔS and ΔSuniverse for this reaction.
Calculate the ΔH, ΔS and ΔSuniverse for this reaction.
Questions in other subjects:

Chemistry, 20.09.2020 17:01


English, 20.09.2020 17:01

English, 20.09.2020 17:01



Mathematics, 20.09.2020 17:01

Mathematics, 20.09.2020 17:01

Biology, 20.09.2020 17:01