Chemistry, 24.03.2020 22:28 mckleinrivero
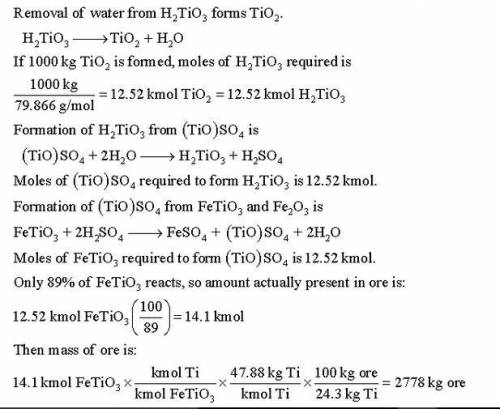
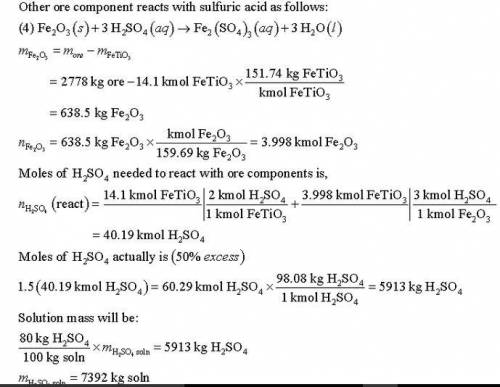
Titanium dioxide (TiO ) is used extensively as a white pigment. It is produced from an ore that contains ilmenite (FeTiO ) and ferric oxide (Fe O ). The ore is digested with an aqueous sulfuric acid solution to produce an aqueous solution of titanyl sulfate [(TiO)SO ] and ferrous sulfate (FeSO ). Water is added to hydrolyze the titanyl sulfate to H TiO , which precipitates, and H SO . The precipitate is then roasted, driving off water and leaving a residue of pure titanium dioxide. (Several steps to remove iron from the intermediate solutions as iron sulfate have been omitted from this description.) Suppose an ore containing 24.3% Ti by mass is digested with an 80% H SO solution, supplied in 50% excess of the amount needed to convert all the ilmenite to titanyl sulfate and all the ferric oxide to ferric sulfate [Fe (SO ) ]. Further suppose that 89% of the ilmenite actually decomposes. Calculate the masses (kg) of ore and 80% sulfuric acid solution that must be fed to produce 1000 kg of pure TiO .

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, vanessa051266
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 07:30, veronica25681
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 09:30, mazielynn84
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

You know the right answer?
Titanium dioxide (TiO ) is used extensively as a white pigment. It is produced from an ore that cont...
Questions in other subjects:

Biology, 27.06.2019 15:10


Mathematics, 27.06.2019 15:10

Mathematics, 27.06.2019 15:10

Chemistry, 27.06.2019 15:10