

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, scottbrandon653
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 02:20, kristieroth1
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 17:30, llamasking
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

Chemistry, 22.06.2019 19:00, andrecoral105
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1
You know the right answer?
At a certain temperature, the equilibrium constant, K c , is 0.00376 for the reaction Cl 2 ( g ) − ⇀...
Questions in other subjects:



Spanish, 18.03.2021 01:50




Mathematics, 18.03.2021 01:50

Social Studies, 18.03.2021 01:50


English, 18.03.2021 01:50

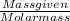
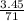
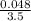 M = 0.013 molar
M = 0.013 molar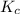 =
= ![\frac{[Cl]^{2} }{[Cl_{2} ]}](/tpl/images/0561/4874/ed9f2.png)


