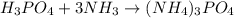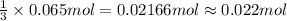Mmonium phosphate NH43PO4 is an important ingredient in many solid fertilizers. It can be made by reacting aqueous phosphoric acid H3PO4 with liquid ammonia. Calculate the moles of ammonium phosphate produced by the reaction of 0.065mol of ammonia. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:30, melikefood01
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 09:00, mercymain1014
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 12:00, hannah2757
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1
You know the right answer?
Mmonium phosphate NH43PO4 is an important ingredient in many solid fertilizers. It can be made by re...
Questions in other subjects:

English, 18.09.2020 01:01

Mathematics, 18.09.2020 01:01

English, 18.09.2020 01:01

Mathematics, 18.09.2020 01:01

Mathematics, 18.09.2020 01:01

Mathematics, 18.09.2020 01:01

Mathematics, 18.09.2020 01:01

Mathematics, 18.09.2020 01:01

English, 18.09.2020 01:01

English, 18.09.2020 01:01