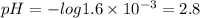Consider a 1.6 × 10-3 M solution of HNO3. Which of the following statements is NOT true? Consider a 1.6 × 10-3 M solution of HNO3. Which of the following statements is NOT true? This solution could dissolve metal. This solution could neutralize a base. This solution would turn litmus to red. This solution has a pH of 11.20. none of the above

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 01:00, jaidencoolman2510
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2

Chemistry, 23.06.2019 01:30, kcarstensen59070
Which statement justifies that hydrogen peroxide (h2o2) is a polar molecule? the o – h bond is nonpolar and the molecule is asymmetric. the o – h bond is nonpolar and the molecule is symmetric. the o – h bond is polar and the molecule is asymmetric. the o – h bond is polar and the molecule is symmetric.
Answers: 1

Chemistry, 23.06.2019 12:00, cooltrey777
How far in advance is weather forecasting most accurate
Answers: 2
You know the right answer?
Consider a 1.6 × 10-3 M solution of HNO3. Which of the following statements is NOT true? Consider a...
Questions in other subjects:

History, 16.10.2020 08:01

Mathematics, 16.10.2020 08:01


Mathematics, 16.10.2020 08:01

Computers and Technology, 16.10.2020 08:01

Mathematics, 16.10.2020 08:01


Mathematics, 16.10.2020 08:01


Mathematics, 16.10.2020 08:01