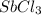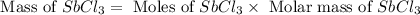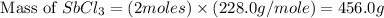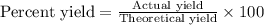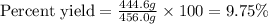Chemistry, 24.03.2020 20:06 brenda0014
Calcula el rendimiento de la reacción entre 2 mol de antonimo metálico(masa atomica 121.76 uma) y 5 mol de cloro(Cl2) (masa molar 70.90) para formar 444.6 G de tricloruro de antonimo (SbCl3) (masa molar 228.0 umal la reaccion quimica es la siguiente 4Sb+6Cl2 = 4SbCl3

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, dimondqueen511
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 07:00, coolkid2041
Calculate the number of moles of ethane in 100 grams
Answers: 3

Chemistry, 22.06.2019 10:30, cheyennecarrillo14
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 12:00, winterblanco
What is the lowest number energy level where a d sublevel is found
Answers: 1
You know the right answer?
Calcula el rendimiento de la reacción entre 2 mol de antonimo metálico(masa atomica 121.76 uma) y 5...
Questions in other subjects:

English, 24.02.2021 18:10



Computers and Technology, 24.02.2021 18:10

Mathematics, 24.02.2021 18:10

Physics, 24.02.2021 18:10

Mathematics, 24.02.2021 18:10



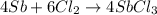
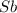 react with 6 mole of
react with 6 mole of 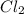
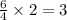 moles of
moles of