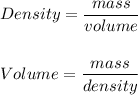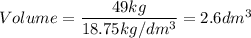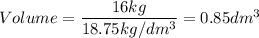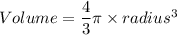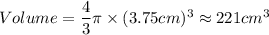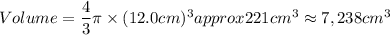Chemistry, 24.03.2020 19:58 AgarioEdit
A particular uranium alloy has a density of 18.75 g/cm3. Please answer the following questions below, providing the explanation to your answers. a. What volume is occupied by a critical mass of 49 kg of this alloy? b. The critical mass can be decreased to 16 kg if the alloy is surrounded by a layer of natural uranium (which acts as a neutron reflector). What is the volume of such smaller mass? Compare your answers to the approximate volumes of a baseball, a volleyball, and a basketball.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, Kjswagout5052
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 10:30, cheyennecarrillo14
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 20:00, bbyjean9974
State one important difference between a physical change and a chemical change?
Answers: 1

Chemistry, 22.06.2019 22:00, aliciaa101
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1
You know the right answer?
A particular uranium alloy has a density of 18.75 g/cm3. Please answer the following questions below...
Questions in other subjects:


Social Studies, 21.11.2019 08:31

Mathematics, 21.11.2019 08:31