

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, luffybunny
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1

Chemistry, 23.06.2019 08:40, sugakookies1
The activation energy for this reaction is 75 kj·mol–1. the enzyme catalase (found in blood) lowers the activation energy to 8.0 kj·mol–1. at what temperature would the non-catalyzed reaction need to be run to have a rate equal to that of the enzyme-catalyzed reaction at 25°c?
Answers: 2
You know the right answer?
2 H2(g) + S2(g) equilibrium reaction arrow 2 H2S(g) At a certain temperature, Kc = 1.30 ✕ 1010 for t...
Questions in other subjects:

Mathematics, 27.09.2019 05:00


History, 27.09.2019 05:00

Mathematics, 27.09.2019 05:00


Chemistry, 27.09.2019 05:00

English, 27.09.2019 05:00

Mathematics, 27.09.2019 05:00

History, 27.09.2019 05:00

Social Studies, 27.09.2019 05:00

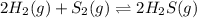
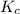 for above equation follows:
for above equation follows:![K_{c}=\frac{[H_2S]^2}{[H_2]^2[S_2]}](/tpl/images/0561/3034/d1522.png)
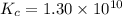
![[H_2]_{eq}=0.00400M](/tpl/images/0561/3034/ad07b.png)
![[S_2]_{eq}=0.00270M](/tpl/images/0561/3034/9642e.png)
![1.30\times 10^{10}=\frac{[H_2S]^2}{(0.00400)^2\times 0.00270}](/tpl/images/0561/3034/e2a23.png)
![[H_2S]_{eq}=23.7,-23.7](/tpl/images/0561/3034/68e27.png)


