
Chemistry, 24.03.2020 19:49 Lovelybunny321
Methylamine, CH3NH2, is a weak base that reacts according to the reaction CH3NH2 + H2O <--> CH3NH3+ + OH- The value of the ionization constant, Kb, is 5.25 x 10 –4. Methylamine reacts to form salts such as methylammonium nitrate, (CH3NH3+)(NO3-). a. Calculate the hydroxide ion concentration, [OH-] of a 0.125 molar aqueous solution of methylamine.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, asims13
Which element in the third period would you expect to have the larger atomic radius, sodium (na) or sulfur (s)? a. sodium, because it has a higher effective nuclear charge attracting electrons in fewer energy levels. b. sodium, because it has fewer protons attracting electrons in the same energy levels. c. sulfur, because it has more protons attracting electrons in more energy levels. d. sulfur, because it has a higher effective nuclear charge attracting electrons in the same energy levels.
Answers: 2

Chemistry, 22.06.2019 04:30, KarenH3512
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1

Chemistry, 22.06.2019 16:00, ghadeeraljelawy
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2
You know the right answer?
Methylamine, CH3NH2, is a weak base that reacts according to the reaction CH3NH2 + H2O <--> CH...
Questions in other subjects:

Mathematics, 21.09.2021 14:00

Chemistry, 21.09.2021 14:00

Mathematics, 21.09.2021 14:00






Mathematics, 21.09.2021 14:00

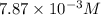
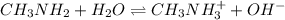
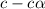
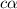
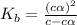
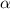 = ?
= ?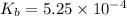
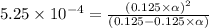
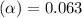
![[OH^-]=c\times \alpha](/tpl/images/0561/1862/0ea5e.png)
![[OH^-]=0.125\times 0.063=7.87\times 10^{-3}M](/tpl/images/0561/1862/08078.png)



