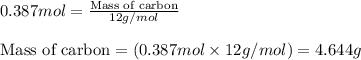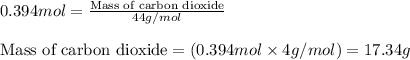For the following reaction, 9.37 grams of carbon (graphite) are allowed to react with 12.6 grams of oxygen gas . carbon (graphite)(s) + oxygen(g) carbon dioxide(g) What is the maximum mass of carbon dioxide that can be formed? grams What is the FORMULA for the limiting reagent? What mass of the excess reagent remains after the reaction is complete? grams Submit AnswerRetry Entire Group

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:50, toniawu18
Problem page gaseous ethane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . if of water is produced from the reaction of of ethane and of oxygen gas, calculate the percent yield of water. be sure your answer has the correct number of significant digits in it.
Answers: 2

Chemistry, 22.06.2019 06:00, applejulianamoreno
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 09:10, aleilyg2005
Select the correct answer from each drop-down menu. describe what happens to a carbon-11 atom when it undergoes positron emission. the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 12:10, kaitlynbernatz2778
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3
You know the right answer?
For the following reaction, 9.37 grams of carbon (graphite) are allowed to react with 12.6 grams of...
Questions in other subjects:


Mathematics, 23.09.2019 08:10


Chemistry, 23.09.2019 08:10



Mathematics, 23.09.2019 08:10



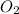 and the mass of excess reagent (carbon) remained is 4.644 grams
and the mass of excess reagent (carbon) remained is 4.644 grams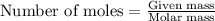 .....(1)
.....(1)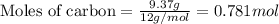
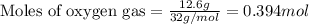
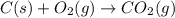
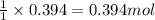 of carbon metal.
of carbon metal.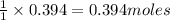 of carbon dioxide
of carbon dioxide