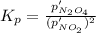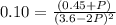Chemistry, 24.03.2020 19:27 lizzyhearts
Nitrogen dioxide is one of the many oxides of nitrogen (often collectively called "NOx") that are of interest to atmospheric chemistry. It can react with itself to form another form of NOx, dinitrogen tetroxide. A chemical engineer studying this reaction fills a 2.0 L flask at 25C with 3.0 atm of nitrogen dioxide gas. He then raises the temperature considerably, and when the mixture has come to equilibrium determines that it contains 2.1 atm of nitrogen dioxide gas. The engineer then adds another 1.5 atm of nitrogen dioxide, and allows the mixture to come to equilibrium again. Calculate the pressure of dinitrogen tetroxide after equilibrium is reached the second time. Round your answer to 2 significant digits.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 01:00, kwarwick0915
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1

Chemistry, 23.06.2019 04:00, onegirl435
The movement of tectonic plates and in two locations is described below: location a: tectonic played push together location b: tectonic plates push apart
Answers: 1
You know the right answer?
Nitrogen dioxide is one of the many oxides of nitrogen (often collectively called "NOx") that are of...
Questions in other subjects:

Spanish, 15.10.2019 15:10

History, 15.10.2019 15:10

Mathematics, 15.10.2019 15:10




Mathematics, 15.10.2019 15:10

History, 15.10.2019 15:10


German, 15.10.2019 15:10

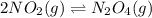
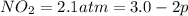

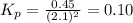
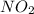 :
: