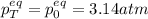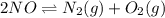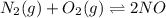Chemistry, 24.03.2020 19:22 brisamauro27
A mixture of 4.76 mol N 2 and 30.45 g NO is heated in a closed vessel to 2000 °C. After heating, the total pressure of the mixture at equilibrium is 3.14 atm . N 2 ( g ) + O 2 ( g ) − ⇀ ↽ − 2 NO ( g ) K p = 0.101 at 2000 ° C In which direction does the reaction proceed after heating to 2000 °C? The reaction is at equilibrium. The reaction proceeds toward the reactants. The reaction proceeds toward the products.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:50, AysiaRamosLee
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 06:00, citlalli30
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 08:30, audrey1256
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 12:00, KKHeffner02
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1
You know the right answer?
A mixture of 4.76 mol N 2 and 30.45 g NO is heated in a closed vessel to 2000 °C. After heating, the...
Questions in other subjects:


Mathematics, 02.02.2021 18:50




Mathematics, 02.02.2021 18:50


Spanish, 02.02.2021 18:50

Mathematics, 02.02.2021 18:50