
For a particular isomer of C8H18, the combustion reaction produces 5113.3 kJ of heat per mole of C8H18(g) consumed, under standard conditions. C8H18(g)+252O2(g)⟶8CO2(g)+9H2O(g)ΔH ∘rxn=−5113.3 kJ/mol What is the standard enthalpy of formation of this isomer of C8H18(g)? ΔH∘f=

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, laurachealsy923
In an energy pyramid, which level has the most available energy?
Answers: 1

Chemistry, 21.06.2019 23:30, ashleyjaslin
Calculate the expected ph values of the buffer systems from the experiments (a, b,c, d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 01:30, kayleg907436
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 02:00, lwattsstudent
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1
You know the right answer?
For a particular isomer of C8H18, the combustion reaction produces 5113.3 kJ of heat per mole of C8H...
Questions in other subjects:



Social Studies, 13.11.2020 04:30


Mathematics, 13.11.2020 04:30

Mathematics, 13.11.2020 04:30

History, 13.11.2020 04:30

History, 13.11.2020 04:30


 is -210.9 kJ
is -210.9 kJ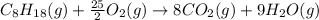
 .
.
![\Delta H^o=[n_{CO_2}\times \Delta H_f^0_{(CO_2)}+n_{H_2O}\times \Delta H_f^0_{(H_2O)}]-[n_{O_2}\times \Delta H_f^0_{(O_2)+n_{C_8H_{18}}\times \Delta H_f^0_{(C_8H_{18})}]](/tpl/images/0561/1035/28ca9.png)
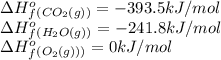
![-511.3kJ/mol=[(8\times -393.5)+(9\times -241.8)]-[(\frac{25}{2}\times 0)+(1\times \Delta H_f^0_{(C_8H_{18})}](/tpl/images/0561/1035/6b6c5.png)



