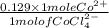Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:20, tenleywood
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 17:40, Snowball080717
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

You know the right answer?
A bottle in lab is labeled [CoCl2.6H2O] = 0.652 M in 8.433 M HCl. If you determine [CoCl42-] to be 0...
Questions in other subjects:

Social Studies, 13.06.2021 09:00

Mathematics, 13.06.2021 09:00


English, 13.06.2021 09:00

Mathematics, 13.06.2021 09:00


Mathematics, 13.06.2021 09:00

Mathematics, 13.06.2021 09:00

Mathematics, 13.06.2021 09:00

Mathematics, 13.06.2021 09:00

![[Co(H_{2}O)_{6}]^{2} + 4Cl^{-} \rightleftharpoons [CoCl_{4}]^{2-} + 6H_{2}O](/tpl/images/0561/0378/1b7cc.png)
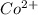 as follows.
as follows.![\frac{0.652 \times 1 mole Co^{2+}}{1 mole [CoCl(H_{2}O)_{6}]^{2+}}](/tpl/images/0561/0378/d9b5b.png)