
Mothballs are composed primarily of the hydrocarbon naphthalene (C10H8)(C10H8). When 1.025 gg of naphthalene is burned in a bomb calorimeter, the temperature rises from 24.25 ∘C∘C to 32.33 ∘C Find ΔErxn for the combustion of naphthalene. The heat capacity of the calorimeter, determined in separate experiment, is 5.11kJ/∘C .

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:00, miguel454545
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2


Chemistry, 22.06.2019 23:00, ceejay8005
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1

Chemistry, 22.06.2019 23:00, tovarclaudia055
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1
You know the right answer?
Mothballs are composed primarily of the hydrocarbon naphthalene (C10H8)(C10H8). When 1.025 gg of nap...
Questions in other subjects:

Chemistry, 07.02.2021 23:40


Mathematics, 07.02.2021 23:40


Health, 07.02.2021 23:40


Mathematics, 07.02.2021 23:40



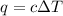
 = change in temperature =
= change in temperature = 



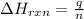
 = enthalpy change of the reaction
= enthalpy change of the reaction


