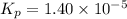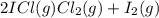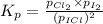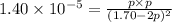Chemistry, 24.03.2020 16:24 faithchambers15
At a temperature below room temperature but with all of the substances still in the gas phase, the equilibrium constant Kp for the decomposition of iodine monochloride (ICl) into I2 and Cl2 is 1.40 × 10–5. If a sealed vessel initially contains 1.70 atm of ICI but no I2 or Cl2. what are the partial pressures of all substances involved in the reaction when it comes to equilibrium?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, dyanaycooper13
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 23.06.2019 03:10, 3jazybraxy
Which is true according to the law of conservation of energy
Answers: 1
You know the right answer?
At a temperature below room temperature but with all of the substances still in the gas phase, the e...
Questions in other subjects:



English, 30.03.2020 12:00

Mathematics, 30.03.2020 12:01



Mathematics, 30.03.2020 12:01

English, 30.03.2020 12:01