Chemistry, 24.03.2020 03:47 HTKPenguin
Calculate the pH of a buffer solution made by adding 15.0 g anhydrous sodium acetate (NaC2H3O2) to 100.0 mL of 0.200 M acetic acid. Assume there is no change in volume on adding the salt to the acid. (pKa for acetic acid is 4.74 or Ka is 1.8 x 10-5)3.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, mercymain1014
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 20:00, 20calzoy
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3

You know the right answer?
Calculate the pH of a buffer solution made by adding 15.0 g anhydrous sodium acetate (NaC2H3O2) to 1...
Questions in other subjects:


Arts, 13.07.2019 09:00

Mathematics, 13.07.2019 09:00

Mathematics, 13.07.2019 09:00


Mathematics, 13.07.2019 09:00

Arts, 13.07.2019 09:00


Mathematics, 13.07.2019 09:00

History, 13.07.2019 09:00

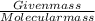
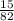
![\frac{[Salt]}{[Acid]}](/tpl/images/0560/4655/6743a.png)