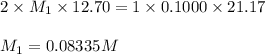Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:40, nadinealonzo6121
Which of the following might a chemist choose to study? a. glacier movement in alaska b. better ways to recycle plastics c. the effects of hurricanes on turtle populations d. the vibrations in bridges caused by big trucks
Answers: 2

Chemistry, 22.06.2019 01:00, jescanarias22
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 09:00, miller5452
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1
You know the right answer?
A solution of malonic acid, H2C3H2O4 , was standardized by titration with 0.1000 M NaOH solution. If...
Questions in other subjects:


Mathematics, 21.01.2021 14:00



Geography, 21.01.2021 14:00

Mathematics, 21.01.2021 14:00

Mathematics, 21.01.2021 14:00

English, 21.01.2021 14:00

English, 21.01.2021 14:00


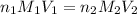
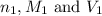 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 
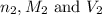 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.