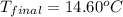Chemistry, 24.03.2020 03:28 bradleylogan78
A student carried heated a 25.00 g piece of aluminum to a temperature of 100°C, and placed it in 100.00 g of water, initially at a temperature of 10.0°C. Determine the final temperature of the system (aluminum and water)
cH2OB4.18J/gc
c Aluminum .900 j/g c

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:00, jrocklove7825
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 17:40, aguilarjose
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1
You know the right answer?
A student carried heated a 25.00 g piece of aluminum to a temperature of 100°C, and placed it in 100...
Questions in other subjects:

Mathematics, 23.01.2022 07:20

Computers and Technology, 23.01.2022 07:20

Mathematics, 23.01.2022 07:20

Social Studies, 23.01.2022 07:20

History, 23.01.2022 07:20





Chemistry, 23.01.2022 07:20

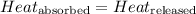
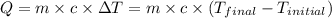
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0560/4279/09236.png) ......(1)
......(1)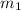 = mass of aluminium = 25.00 g
= mass of aluminium = 25.00 g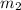 = mass of water = 100 g
= mass of water = 100 g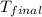 = final temperature = ?°C
= final temperature = ?°C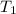 = initial temperature of aluminium = 100°C
= initial temperature of aluminium = 100°C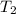 = initial temperature of water = 10°C
= initial temperature of water = 10°C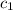 = specific heat of aluminium = 0.900 J/g°C
= specific heat of aluminium = 0.900 J/g°C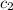 = specific heat of water= 4.18 J/g°C
= specific heat of water= 4.18 J/g°C![25\times 0.900\times (T_{final}-100)=-[100\times 4.18\times (T_{final}-10)]](/tpl/images/0560/4279/4bd6d.png)