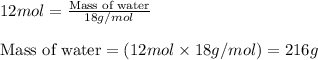Chemistry, 24.03.2020 02:44 musicaljay1276
The balanced chemical equation for the combustion of propane is C3H8(g)+5O2(g) --> 3CO2(g)+4H2O(g) Which statement is correct about the complete combustion of 3.00 mole of propane, C3H8? \rm C_3H_8(g) + 5 O_2(g) --> 3 CO_2(g) + 4 H_2O(g)Which statement is correct about the complete combustion of 3.00 mole of propane, \rm C_3H_8?1. 12.00 mol H2O are produced.2. 3.00 g CO2 are produced.3. 3.00 mol CO2 are produced.4. 12.00 g H2O are produced

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:50, mrylenastewart
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1


Chemistry, 22.06.2019 08:00, wizz4865
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2
You know the right answer?
The balanced chemical equation for the combustion of propane is C3H8(g)+5O2(g) --> 3CO2(g)+4H2O(g...
Questions in other subjects:



Mathematics, 30.08.2019 16:30


Computers and Technology, 30.08.2019 16:30


Mathematics, 30.08.2019 16:30



Social Studies, 30.08.2019 16:30

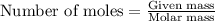 ......(1)
......(1)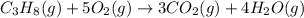
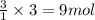 of carbon dioxide
of carbon dioxide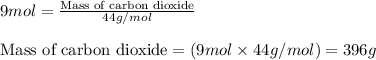
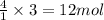 of water
of water