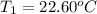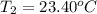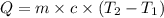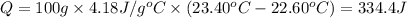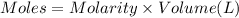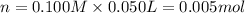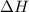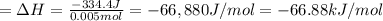Chemistry, 24.03.2020 00:29 PrincesssOfficial
In a coffe cup calorimeter, 50.0mL of 0.100M of AgNO3 and 50mL of 0.100M HCl are mixed to yield the following reaction:
Ag+ (aq) + Cl -==> AgCl(s)
The two solutions were initially at 22.60°C, and the final temperature is 23.40°C. How do I calculate the heat that accompanies this reaction in kJ/mol, assuming that the combined solution has a mass of 100g and a specific heat capacity of 4.18 J/g°C.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:30, kiki197701
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 22.06.2019 21:00, nsutton9985
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1
You know the right answer?
In a coffe cup calorimeter, 50.0mL of 0.100M of AgNO3 and 50mL of 0.100M HCl are mixed to yield the...
Questions in other subjects: