
Chemistry, 24.03.2020 00:00 paolaviviana
Hydrogen sulfide decomposes according to the following reaction, for which Kc = 9.30 10-8 at 700°C. 2 H2S(g) 2 H2(g) + S2(g) If 0.31 mol H2S is placed in a 4.1 L container, what is the equilibrium concentration of H2(g) at 700°C?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:40, kellypechacekoyc1b3
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 04:00, soonerlady19
Which atom or ion is the largest? 0 a. 0 0 0 0 e. li
Answers: 2

Chemistry, 22.06.2019 05:30, jzjajsbdb8035
Which other elements contain the same number of outer electrons as sodium
Answers: 3

You know the right answer?
Hydrogen sulfide decomposes according to the following reaction, for which Kc = 9.30 10-8 at 700°C....
Questions in other subjects:

Mathematics, 08.11.2020 06:50

Mathematics, 08.11.2020 06:50

Mathematics, 08.11.2020 06:50


Mathematics, 08.11.2020 07:00

Social Studies, 08.11.2020 07:00



English, 08.11.2020 07:00

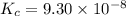 }
}![[concentration]=\frac{moles}{volume (L)}](/tpl/images/0559/9416/7e1bc.png)
![[H_2S]=\frac{0.31 mol}{4.1 L}=0.076 M](/tpl/images/0559/9416/b2f14.png)
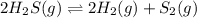
![K_c=\frac{[H_2]^2[S_2]}{[H_2S]^2}](/tpl/images/0559/9416/3ac5e.png)
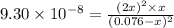
![[H_2]=2x=2\times 0.00051 M=0.0010 M](/tpl/images/0559/9416/46b67.png)



