Given the following reaction:
Mg(OH)2 + 2HCI -> MgCl2 + 2H2O
How many mol...

Chemistry, 23.03.2020 23:54 onlymyworld27
Given the following reaction:
Mg(OH)2 + 2HCI -> MgCl2 + 2H2O
How many moles of MgCl, will be produced from 32.0 g of Mg(OH)2, assuming HCl is available in excess?
moles (round to three significant figures)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, falishaduncanovmtz2
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 09:10, GreatBaconGamer
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1


Chemistry, 22.06.2019 15:00, tcapele252
‘which reaction would most likely require the use of an inert electrode?
Answers: 1
You know the right answer?
Questions in other subjects:



English, 12.09.2021 08:30


English, 12.09.2021 08:30

Mathematics, 12.09.2021 08:30

Chemistry, 12.09.2021 08:30

Physics, 12.09.2021 08:30


Mathematics, 12.09.2021 08:30

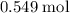 (three significant figures.)
(three significant figures.) 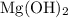 :
: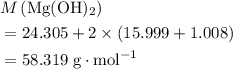 .
.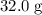 of
of 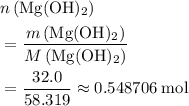 .
.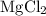 . In other words, for each mole of
. In other words, for each mole of  .
.

