
A 18.08-g sample of the ionic compound , where is the anion of a weak acid, was dissolved in enough water to make 116.0 mL of solution and was then titrated with 0.140 M . After 500.0 mL was added, the pH was 4.63. The experimenter found that 1.00 L of 0.140 M was required to reach the stoichiometric point of the titration. a What is the molar mass of ? Molar mass = 129.14 g/mol b Calculate the pH of the solution at the stoichiometric point of the titration.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, megaaan214p61pb7
Which compounds have the empirical formula ch2o? a. c2h4o2 b. c3h6o3 c. ch2o2 d. c5h10o5 e. c6h12o6
Answers: 3



Chemistry, 22.06.2019 14:30, clemsongirl5392
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2
You know the right answer?
A 18.08-g sample of the ionic compound , where is the anion of a weak acid, was dissolved in enough...
Questions in other subjects:

Mathematics, 10.09.2020 15:01

Mathematics, 10.09.2020 15:01

Mathematics, 10.09.2020 15:01

Mathematics, 10.09.2020 15:01

English, 10.09.2020 15:01

Mathematics, 10.09.2020 15:01

Mathematics, 10.09.2020 15:01

Mathematics, 10.09.2020 15:01

Mathematics, 10.09.2020 16:01

Mathematics, 10.09.2020 16:01

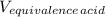 = 1.00 L
= 1.00 L
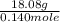
![10^{-4.63]](/tpl/images/0559/8018/9ece0.png)

![[A^-]equ = \frac{0.140M*1.00L}{1.00L+0.116L}](/tpl/images/0559/8018/a9cf4.png)
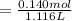
 of HA =
of HA = 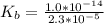
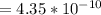
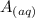 +
+ 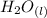
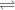
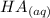 +
+ 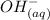
![K_b = \frac{[HA][OH^-]}{[A^-]}](/tpl/images/0559/8018/12da8.png)
![4.35*10^{-10} = \frac{[x][x]}{[0.1255-x]}](/tpl/images/0559/8018/ab7b7.png)
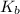 is very small, (o.1255 - x) = 0.1255
is very small, (o.1255 - x) = 0.1255
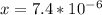
 ]
]

