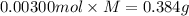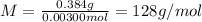A sample of a monoprotic acid (HA) weighing 0.384 g is dissolved in water and the solution is titrated with aqueous NaOH. If 30.0 mL of 0.100 M NaOH is required to reach the equivalence point, what is the molar mass of HA?
(a) 211 g/mol
(b) 128 g/mol
(c) 81.0 g/mol
(d) 37.0 g/mol
(e) 20.3 g/mol

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:30, kayleg907436
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 02:30, ethanmel21
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 23.06.2019 01:30, joyelewis58
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1
You know the right answer?
A sample of a monoprotic acid (HA) weighing 0.384 g is dissolved in water and the solution is titrat...
Questions in other subjects:










Mathematics, 30.08.2019 10:00

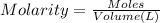
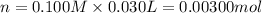
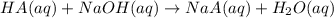
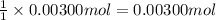 of HA
of HA