
Chemistry, 23.03.2020 21:45 angelina12386
For cobalt, Co, the heat of vaporization at its normal boiling point of 3097 °C is 389.1 kJ/mol. The entropy change when 1.85 moles of liquid Co vaporizes at 3097 °C, 1 atm is J/K.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:00, montimcdaniel
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1



Chemistry, 23.06.2019 04:00, izzyp619
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3
You know the right answer?
For cobalt, Co, the heat of vaporization at its normal boiling point of 3097 °C is 389.1 kJ/mol. The...
Questions in other subjects:

Arts, 02.03.2020 21:03

Computers and Technology, 02.03.2020 21:03


Biology, 02.03.2020 21:03






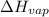 =
= 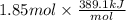
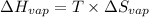
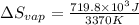
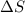 for vaporization is 213.6 J/K.
for vaporization is 213.6 J/K.

