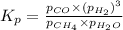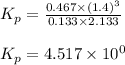Chemistry, 23.03.2020 21:09 darkskinnednune
Steam reforming of methane ( CH4 ) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. An industrial chemist studying this reaction fills a 1.5 L flask with 0.60 atm of methane gas and 2.6 atm of water vapor at 47. °C. He then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of hydrogen gas to be 1.4 atm. Calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. Round your answer to 2 significant digits x10.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:00, chefdnguyen
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 13:30, ayoismeisalex
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1

Chemistry, 22.06.2019 19:10, asdfghhk9805
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2
You know the right answer?
Steam reforming of methane ( CH4 ) produces "synthesis gas," a mixture of carbon monoxide gas and hy...
Questions in other subjects:



Mathematics, 05.10.2019 02:00

Mathematics, 05.10.2019 02:00


Mathematics, 05.10.2019 02:00

Spanish, 05.10.2019 02:00



Mathematics, 05.10.2019 02:00


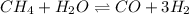
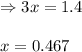
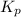 for above equation follows:
for above equation follows: