
A 100mL reaction vessel initially contains 2.60x10^-2 moles of NO and 1.30x10^-2 moles of H2. At equilibrium the concentration of NO in the vessel is 0.161M. At equilibrium the vessel contains N2, H2O and H2. What is the value of the equilibrium constant Kc for the following reactions?2H2(g) + 2NO(g) <---> 2 H2O(g) + N2(g)

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:00, shayneseaton
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 17:50, mytymikey123
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2
You know the right answer?
A 100mL reaction vessel initially contains 2.60x10^-2 moles of NO and 1.30x10^-2 moles of H2. At equ...
Questions in other subjects:

Biology, 28.10.2020 05:20

English, 28.10.2020 05:20

English, 28.10.2020 05:20


Mathematics, 28.10.2020 05:20

Social Studies, 28.10.2020 05:20

English, 28.10.2020 05:20

Mathematics, 28.10.2020 05:20

Mathematics, 28.10.2020 05:20

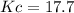

![[H_2]_0=\frac{1.30x10^{-2}mol}{0.1L}=0.130M](/tpl/images/0559/4541/78a1b.png)
![[NO]_0=\frac{2.60x10^{-2}mol}{0.1L}=0.260M](/tpl/images/0559/4541/9b7c0.png)
 , due to the chemical reaction extent, turns out:
, due to the chemical reaction extent, turns out:![x=\frac{[NO]_{0}-[NO]_{eq}}{2} =\frac{0.260M-0.161M}{2}=0.049M](/tpl/images/0559/4541/4c52a.png)
![[H_2]_{eq}=0.130M-2(0.049M)=0.032M](/tpl/images/0559/4541/cb7ca.png)
![[H_2O]_{eq}=2(0.049M)=0.098M](/tpl/images/0559/4541/63a65.png)
![[N_2]_{eq}=0.049M](/tpl/images/0559/4541/f2124.png)
![Kc=\frac{[H_2O]_{eq}^2[N_2]_{eq}}{[H_2]_{eq}^2[NO]_{eq}^2}=\frac{(0.098M)^2(0.049M)}{(0.032M)^2(0.161M)^2} \\\\Kc=17.7](/tpl/images/0559/4541/1d534.png)


