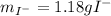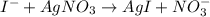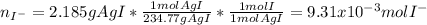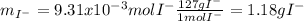Chemistry, 23.03.2020 20:42 klmklm3799
A soluble iodide was dissolved in water. Then an excess of silver nitrate, AgNO3, was added to precipitate all of the io- dide ion as silver iodide, AgI. If 1.545 g of the soluble iodide gave 2.185 g of silver iodide, how many grams of iodine are in the sample of soluble iodide

Answers: 3


Other questions on the subject: Chemistry




Chemistry, 23.06.2019 10:30, taniyahbenyamin2
What’s the physical properties in calcium chloride
Answers: 1
You know the right answer?
A soluble iodide was dissolved in water. Then an excess of silver nitrate, AgNO3, was added to preci...
Questions in other subjects:






English, 29.10.2021 14:00


Social Studies, 29.10.2021 14:00