
Chemistry, 23.03.2020 20:21 ranaawilliamsoowl6dk
Consider the reaction represented by the equation?2SO2(g) + O2(g) 2SO3(g).?For the system at chemical equilibrium, which of the following explains what happens after the volume of the reaction mixture is increased (assume constant temperature)?
a. The amount of SO3(g) increases and the value for K increases.
b. The amount of SO3(g) decreases and the value for K increases.
c. The amount of SO3(g) stays the same and the value for K decreases.
d. The amount of SO3(g) decreases and the value for K stays the same.
e. The amount of SO3(g) increases and the value for K stays the same

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:40, aaliyahthomas37
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1


Chemistry, 23.06.2019 09:30, sharmadaman641
What is the best describtion of the side of the moon that faces earth?
Answers: 2
You know the right answer?
Consider the reaction represented by the equation?2SO2(g) + O2(g) 2SO3(g).?For the system at chemica...
Questions in other subjects:


Mathematics, 16.10.2019 09:10


Biology, 16.10.2019 09:10

Physics, 16.10.2019 09:10

Mathematics, 16.10.2019 09:10



Mathematics, 16.10.2019 09:10

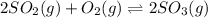

![K=\frac{[SO_3]_{eq}^2}{[SO_2]_{eq}^2[O_2]_{eq}}](/tpl/images/0559/3324/74387.png)


