
Cerium (IV) ions are strong oxidizing agents in acid ic solution, oxidizing arsenio us acid to arsen ic acid accor ding to the following equa tion:
2Ce4+(aq)+H3AsO3(aq)+3H2O(l)→2Ce3+( aq)+H3AsO4(aq)+2H+(aq)
A sample of As2O3 weighing 0.217 g is dissolved in basic solution and then acidified to make H3AsO3. Its titration with a solution of acidic cerium{IV) sulfate requires 21.47 ml. Determine the original concentration of Ce^4+(aq) in the titrating solution

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:10, carsonjohnsonn
Can smoke be transformed into liquid or used as energy or both?
Answers: 2


Chemistry, 22.06.2019 14:30, villarrealc1987
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 15:30, lizzyhearts
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3
You know the right answer?
Cerium (IV) ions are strong oxidizing agents in acid ic solution, oxidizing arsenio us acid to arsen...
Questions in other subjects:

English, 07.07.2019 04:00

Biology, 07.07.2019 04:00

History, 07.07.2019 04:00

Social Studies, 07.07.2019 04:00

Mathematics, 07.07.2019 04:00



English, 07.07.2019 04:00

Physics, 07.07.2019 04:00

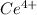 (aq) in the titrating solution.
(aq) in the titrating solution.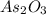 = 0.217 g
= 0.217 g
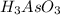 have 1 mole of As.
have 1 mole of As.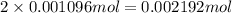 of
of 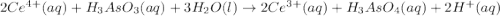
 of cerium (IV) ions.
of cerium (IV) ions.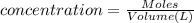
![[Ce^{4+}]=\frac{0.004384 mol}{0.02147 L}=0.2042 M](/tpl/images/0559/2089/fb855.png)



