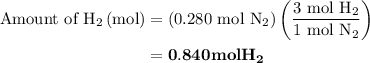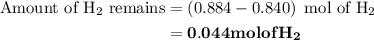Chemistry, 16.01.2020 10:31 balletbella0531
Nitrogen and hydrogen combine at high temperature, in the presence of a catalyst, to produce ammonia. n2(g)+3h2(g)=2nh3(g). assume 0.280 mol of n2 and 0.884 mol of h2 are present initially.. after complete reaction, how many moles of ammonia are . how many moles of h2 . how many moles of n2, what is the limiting or hydrogen)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, andrejr0330jr
What is the molar mass of potassium nitrate, kno3
Answers: 1

Chemistry, 22.06.2019 11:30, chelseychew32
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2


Chemistry, 22.06.2019 17:30, kevin72937
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1
You know the right answer?
Nitrogen and hydrogen combine at high temperature, in the presence of a catalyst, to produce ammonia...
Questions in other subjects:


Health, 23.05.2020 01:02

Physics, 23.05.2020 01:02

Mathematics, 23.05.2020 01:02




Mathematics, 23.05.2020 01:02


Mathematics, 23.05.2020 01:02

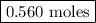
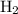 remain on completion of reaction is
remain on completion of reaction is 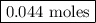
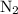 remain on completion of reaction is
remain on completion of reaction is 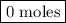
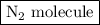
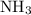 is as follows:
is as follows: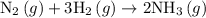
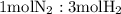
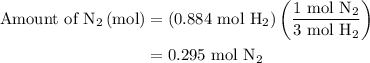
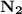 is present in limited quantity and is a limiting reagent.
is present in limited quantity and is a limiting reagent.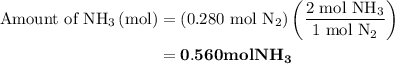
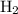 , therefore, the number of moles of
, therefore, the number of moles of