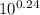Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, maryjane8872
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1

Chemistry, 22.06.2019 21:00, Janznznz4012
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3

Chemistry, 23.06.2019 00:00, jasmin5285
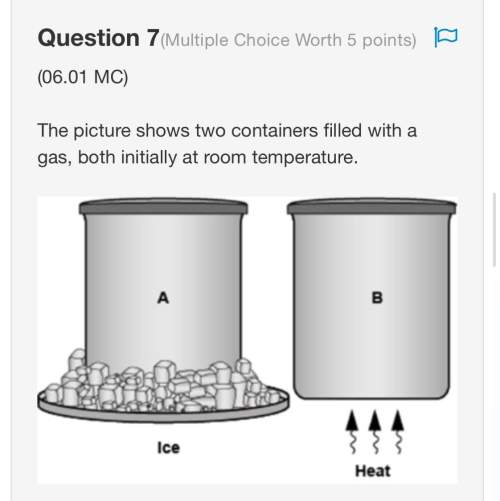
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1
You know the right answer?
A buffer at pH 7.45 is prepared by mixing solutions of KH2PO4 and K2HPO4. Which of the following rat...
Questions in other subjects:

English, 23.06.2019 14:00

Mathematics, 23.06.2019 14:00


English, 23.06.2019 14:00


History, 23.06.2019 14:00

Mathematics, 23.06.2019 14:00

Physics, 23.06.2019 14:00


![\frac{[Base]}{[Acid]}](/tpl/images/0559/1224/ebefd.png) = 1.737
= 1.737