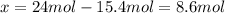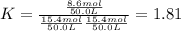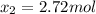While ethanol (CH3CH2OH is produced naturally by fermentation, e. g. in beer- and wine-making, industrially it is synthesized by reacting ethylene CH2CH2) with water vapor at elevated temperatures. A chemical engineer studying this reaction fills a 50.0 L tank at 22. °C with 24. mol of ethylene gas and 24. mol of water vapor. He then raises the temperature considerably, and when the mixture has come to equilibrium determines that it contains 15.4 mol of ethylene gas and 15.4 mol of water vapor The engineer then adds another 12. mol of water, and allows the mixture to come to equilibrium again. Calculate the moles of ethanol after equilibrium is reached the second time. Round your answer to 2 significant digits.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, eborkins
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 04:30, terrancebest
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2


Chemistry, 22.06.2019 20:00, bettybales1986
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2
You know the right answer?
While ethanol (CH3CH2OH is produced naturally by fermentation, e. g. in beer- and wine-making, indus...
Questions in other subjects:

History, 08.12.2020 01:10


Mathematics, 08.12.2020 01:10



English, 08.12.2020 01:10

Mathematics, 08.12.2020 01:10


English, 08.12.2020 01:10

English, 08.12.2020 01:10


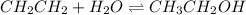
![K=\frac{[CH_3CH_2OH]_{eq}}{[CH_2CH_2]_{eq}[H_2O]_{eq}}](/tpl/images/0559/0707/4b24a.png)
 result:
result: