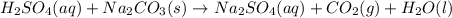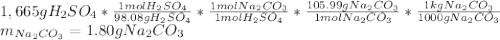Chemistry, 23.03.2020 16:51 tryintopassenioryear
A bottle containing 1,665 g of sulfuric acid (H2SO4, 98.08 g/mol) was spilled in a laboratory. The emergency spill kit contained a full 2.0 kg bottle of sodium carbonate (105.99 g/mol). Is this enough sodium carbonate to neutralize the acid, according to the following reaction
H2SO4(aq) + Na2CO3(s) → Na2SO4(aq) + CO2(g) + H2O(l)
A. Yes, there is more than enough sodium carbonate.
B. Yes, there is exactly enough sodium carbonate-but no excess.
C. No, there is not enough sodium carbonate, but the amount is only about 10% too small.
D. No, there is not nearly enough sodium carbonate.
E. No, the reaction will start going backwards.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, Brittpaulina
Based on the law of conservation of energy, which statement is false? answer- energy is lost when machines dont work right
Answers: 1

Chemistry, 21.06.2019 23:00, cami30031cami3003
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2

Chemistry, 22.06.2019 06:30, Pizzapegasus1
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 07:30, avisconti571
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2
You know the right answer?
A bottle containing 1,665 g of sulfuric acid (H2SO4, 98.08 g/mol) was spilled in a laboratory. The e...
Questions in other subjects:



Mathematics, 08.03.2021 17:50

History, 08.03.2021 17:50

Medicine, 08.03.2021 17:50


Mathematics, 08.03.2021 17:50

Mathematics, 08.03.2021 17:50