Chemistry, 23.03.2020 16:58 mistiehaas
A 850.0 mL cylinder containing B2H6 at a pressure of 0.900 atm is connected by a valve to 1000 mL cylinder containing C6H6 at 608.0 torr pressure. Calculate the partial pressure (torr) of C6H6 when the valve is opened.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, jwood287375
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3


Chemistry, 22.06.2019 09:20, lanaiheart7
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

You know the right answer?
A 850.0 mL cylinder containing B2H6 at a pressure of 0.900 atm is connected by a valve to 1000 mL cy...
Questions in other subjects:


History, 15.12.2021 08:30



Mathematics, 15.12.2021 08:30

Mathematics, 15.12.2021 08:30



Mathematics, 15.12.2021 08:30

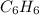 when the valve is opened is 328.6 Torr.
when the valve is opened is 328.6 Torr.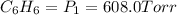

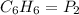
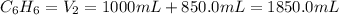
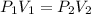 ( Boyle's law)
( Boyle's law)