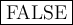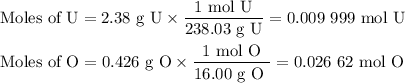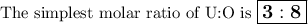2.38 grams of uranium is heated in a current of air. The resulting oxide
weighs 2.806 grams. W...

Chemistry, 23.03.2020 02:14 selenaK9514
2.38 grams of uranium is heated in a current of air. The resulting oxide
weighs 2.806 grams. When solving for empirical formula, the simplest
molar ratio of uranium is 1.
TRUE or
FALSE

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 23:30, treylartigue
The appropriate concentration for an iodine sanitizer is
Answers: 1

Chemistry, 23.06.2019 01:00, aliviadushane
If a straight-chain hydrocarbon is a gas at room temperature, how many carbon atoms will it have? a. 6 carbon atoms b. 12 carbon atoms c. 24 carbon atoms d. 3 carbon atoms
Answers: 1
You know the right answer?
Questions in other subjects:





Mathematics, 08.02.2021 23:10




Mathematics, 08.02.2021 23:10