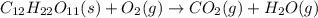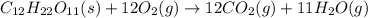Chemistry, 22.03.2020 06:18 jordynj6363
Predict the products for the reaction of burning sucrose, C12H22O11(s), with excess oxygen, O2(g)
(A) 6CO(g), 6CO2(g), 11H2O(g
B)12CO(g), 11H2O(g)
C)12CO2(g), 11H2O(g)
D)24CO2(g), 22H2O(g)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:30, martinez6221
Forests and meadows are often cut down to make way for farms or large number of new homes. what are some of the elements of ecosystems that are lost when plants in these areas are removed?
Answers: 2

Chemistry, 21.06.2019 17:40, Calumworthy6046
What are the resulting coefficients when you balance the chemical equation for the combustion of ethane, c2h6? in this reaction, ethane is burned in the presence of oxygen (o2) to form carbon dioxide (co2) and water (h2o). (g)+(g)→(g)+(g)
Answers: 1


Chemistry, 22.06.2019 22:30, itsmaddierae11
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1
You know the right answer?
Predict the products for the reaction of burning sucrose, C12H22O11(s), with excess oxygen, O2(g)
Questions in other subjects:

Mathematics, 26.08.2019 05:20


Chemistry, 26.08.2019 05:20

Social Studies, 26.08.2019 05:20


History, 26.08.2019 05:20



Mathematics, 26.08.2019 05:20