
Chemistry, 22.03.2020 05:00 Adolfosbaby
Calculate the maximum numbers of moles and grams of iodic acid (HIO3) that can form when 341 g of iodine trichloride reacts with 151.7 g of water:
ICl3 + H2O → ICl + HIO3 + HCl [unbalanced]

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 07:00, asims13
The following transition occurs at a molecular level for a substance. what transition corresponds to this change in microscopic structure? the carbon dioxide molecules on the left are in a regular, tightly packed pattern. after heating, it becomes much lower density. a. melting b. boiling c. sublimation d. freezing
Answers: 1


Chemistry, 23.06.2019 09:20, weridness80
Which of the following occurs along coasts during the day?
Answers: 3
You know the right answer?
Calculate the maximum numbers of moles and grams of iodic acid (HIO3) that can form when 341 g of io...
Questions in other subjects:


Mathematics, 16.12.2020 17:30





History, 16.12.2020 17:30

Social Studies, 16.12.2020 17:30

Mathematics, 16.12.2020 17:30

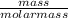
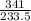 = 1.46moles
= 1.46moles 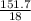 = 8.43moles
= 8.43moles 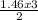 = 2.19 moles
= 2.19 moles 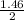 = 0.73moles
= 0.73moles 

